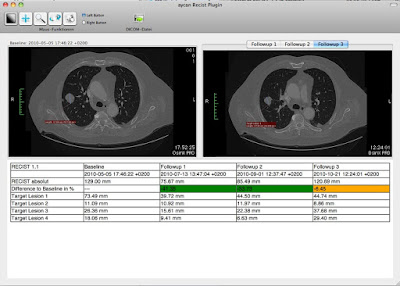
aycan releases another clinical application plugin for OsiriX PRO. The AYRecist Plugin is for the evaluation of oncological followups according to the RECIST 1.1 guidelines.
The AYRecist Plugin supports the user with an unlimited number of followups. After definition of the Target Lesions and Non-Target Lesions by the user it will display baseline and followup lesion synchronized. The AYRecist Plugin also shows a table of the RECIST parameters and the response over time. The key images and the table can be embeded into a report and exported as DICOM files to the PACS.
The AYRecist plugin is available at the end of March 2011 in Europe as option for the CE-labeled OsiriX PRO.
The software was presented during the 4th ECR Workstation Face-off at ECR 2011 in Vienna.
About RECIST:
RECIST (Response Evaluation Criteria In Solid Tumors) is a set of published rules that define when cancer patients improve (“respond”), stay the same (“stable”) or worsen (“progression”) during treatments.
The original criteria were published in February 2000 by an international collaboration including the European Organization for Research and Treatment of Cancer (EORTC), National Cancer Institute (NCI) of the United States and the National Cancer Institute of Canada Clinical Trials Group.
RECIST 1.1, published in January 2009, is an update to the original criteria. Today, the majority of clinical trials evaluating cancer treatments for objective response in solid tumors are using RECIST.
(Source www.recist.com)